Vision is the result of multiple biochemical reactions that happen within the eye structure. In this process, the photoreceptors are in charge of a phenomenon called phototransduction: detection of the photons of light and the absorption by their photopigment to initiate the transmission of the visual signal towards the brain.
The visual pigment responsible for phototransduction in vertebrate species is called rhodopsin. It is present in the external part of the photoreceptors that form the retina, which undergo structural changes leading to their photobleaching when exposed to photons of light. [1]
Rhodopsin is in maximal density when the eye is under dark conditions and in minimal density (photobleached) when the eye is exposed to illumination. Moreover, it has a peak absorption around 500 nm which shifts upon photobleaching [2]. This characteristic is exploited by imaging techniques for clinical purposes, to obtain information about the rhodopsin concentration due to its implication in different eye diseases such as age-related macular degeneration or retinitis pigmentosa.
Ultrafast Retinal Densiometry: basic principles
Retinal Densiometry, or Fundus Reflection Densiometry is a technique that allows for the non-invasive monitoring of the eye´s optical response to visual stimuli. [3] It is a well-established technique that has been a matter of studies for more than 50 years which uses reflectometry to compare the light that is reflected from an illuminated retina, meaning minimal visual pigment (photobleached); with the light that is reflected back from an eye that has maximum visual pigment (under dark conditions); hence giving an idea of the photopigment optical density.
When the visual pigment absorbs one photon, it induces its photoisomerization, followed by the initiation of a photo-cycle where rhodopsin passes through different intermediate structural states until it is regenerated back again. During these three stages, the absorption peak changes from 500 nm, to 570 nm after the photoisomerization, to 450 nm when the rhodopsin is completely bleached [4]. This spectral absorption shift is thus used to measure the functionality of the eye photoreceptors and light absorption from intermediate states should be minimized for the precise quantitative mapping of rod photoreceptors functioning.
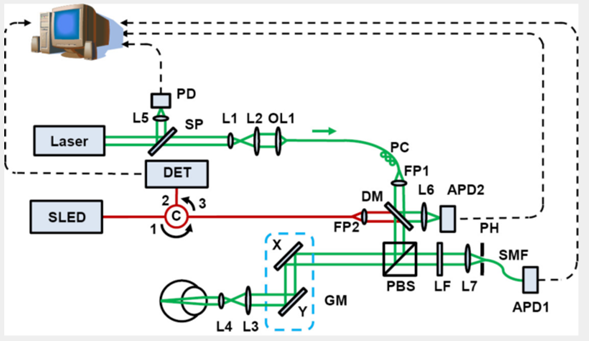
This principle is used in combination with multiple techniques including Scanning Laser Ophthalmoscopy (SLO), fundus photography, or Optical Coherence Tomography (OCT). An example is shown in Figure 1, where an experimental setup of an SLO system is shown. These methods use pulsed laser illumination in order to improve the signal-to-noise ratio and resolution of the images, by reducing the possibility of having absorption from rhodopsin intermediates [4]. Moreover, due to the absorption peaks of the rhodopsin in the VIS range, lasers with emission within the absorption peaks of the pigment are required.
In this scope, Superluminescent Diodes (SLEDs) have been proposed previously by several authors, for broadband illumination in the VIS and NIR range, at low power levels of several uW. [2] [4] Others have considered the use of different single linewidth lasers or more compact alternatives including white light lasers [5].
4292.2014.11.06. PMID: 25694955; PMCID: PMC4312301.)
Furthermore, more recent approaches for ultrafast retinal densiometry combine the above mentioned systems principles with the use of Adaptive Optics. AO for biomedical applications has been used for more than 25 years to study the structures of living biological tissues due to its capability of accounting for the optical aberrations. When applied to optical densiometry, an imaging device is able to dynamically correct the eye ́s aberrations produced in the anterior part of the eye and excite the retinal photoreceptors with sub-cellular resolution in vivo. [3]
Iceblink Supercontinium Laser for Ultrafast Retinal Densiometry
In previous chapters, the versatility of the Iceblink supercontinuum laser was studied for multiple biomedical applications. This time, we propose the use of our high-quality light source for Ultrafast Retinal Densiometry.
Some key features of the Iceblink that will make your experimental setup for retinal imaging a next-level system include:
• Picosecond pulses that match the isomerization time scales of visual pigment during phototransduction.
• Broad illumination in the VIS range. The Iceblink offers and excellent spectral coverage of the different absorption peaks of visual pigment upon the different conformational changes during the measurements.
• The combination of the Iceblink with its tunable module called Boreal allows for the selection of wavelengths in the VIS spectra, meaning possibility to adjust the emission wavelength at 500, 570 or 450 nm with a minimum resolution of 10 nm.
• The average power of >3W ensures excitation of the photoreceptors within the eye structure. The power of the Iceblink in combination with the Boreal at specific wavelengths ranging from μW to several mW allows for damage-free measurements of biological tissues.
References
[1] Bedggood P, Britten-Jones AC, Ayton LN, Metha A. Assessment of photoreceptor function with ultrafast retinal densitometry. Biomed Opt Express. 2022 Sep 13;13(10): 5311 – 5326. doi: 10.1364/BOE.472174. PMID: 36425640; PMCID: PMC9664880.
[2] Liu T, Liu X, Wen R, Lam BL, Jiao S. In vivo imaging rhodopsin distribution in the photoreceptors with nano-second pulsed scanning laser ophthalmoscopy. Quant Imaging Med Surg. 2015 Feb;5(1):63-8. doi: 10.3978/j.issn.2223-4292.2014.11.06. PMID: 25694955; PMCID: PMC4312301.
[3] Liem AT, Keunen JE, Van Norren D. Clinical applications of fundus reflection densitometry. Surv Ophthalmol. 1996 Jul-Aug;41(1):37-50. doi: 10.1016/s0039-6257(97)81994-7. PMID: 8827929.
[4] Phillip Bedggood,Andrew Metha; Variability in Bleach Kinetics and Amount of Photopigment between Individual Foveal Cones.Invest. Ophthalmol. Vis. Sci. 2012;53(7): 3673 – 3681. https://doi.org/10.1167/iovs.11-8796.
[5] Bedggood P, Metha A. Optical imaging of human cone photoreceptors directly following the capture of light. PLoS One. 2013 Nov 15;8(11):e79251. doi:10.1371/journal.pone.0079251. PMID: 24260177; PMCID: PMC3829831.