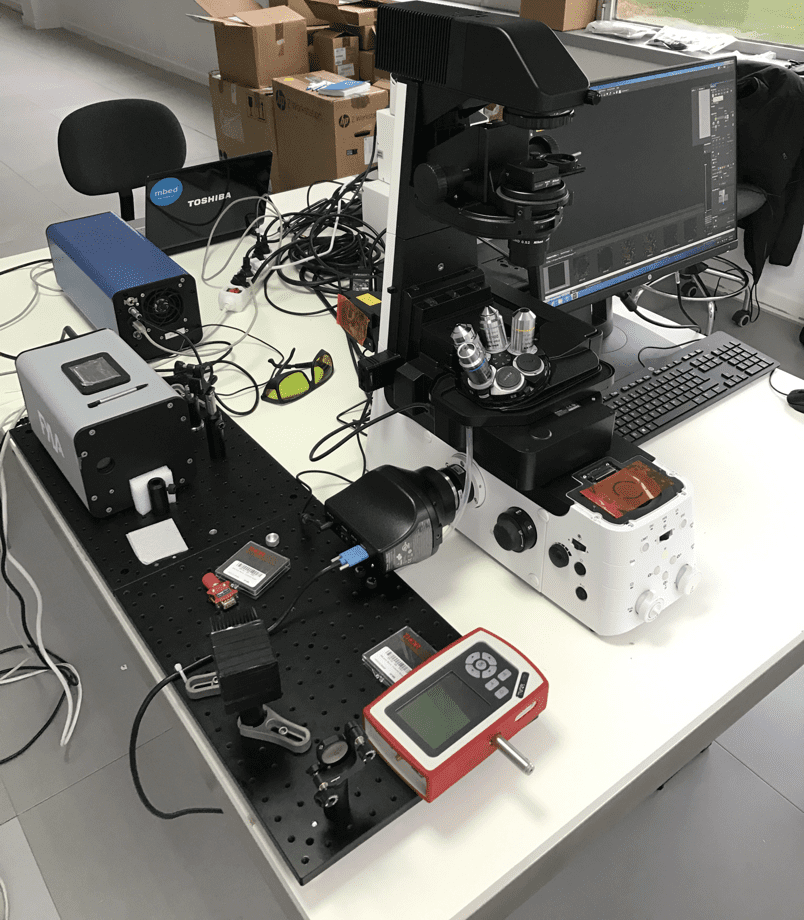
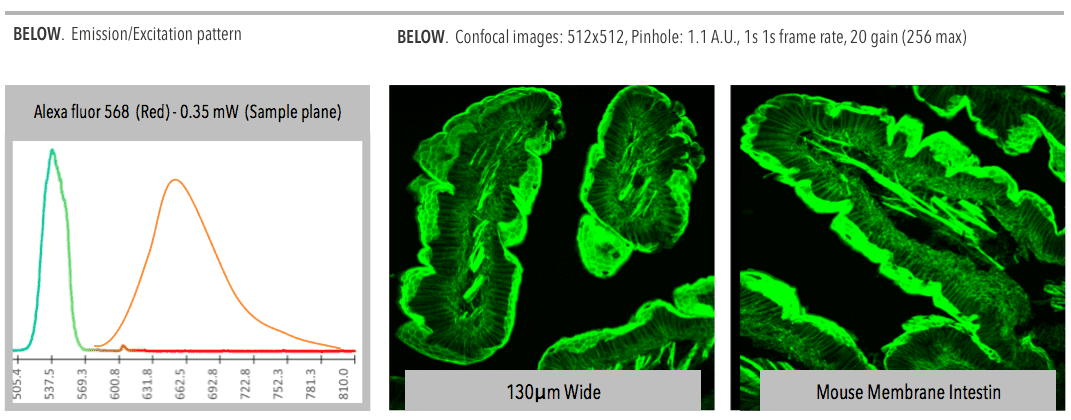
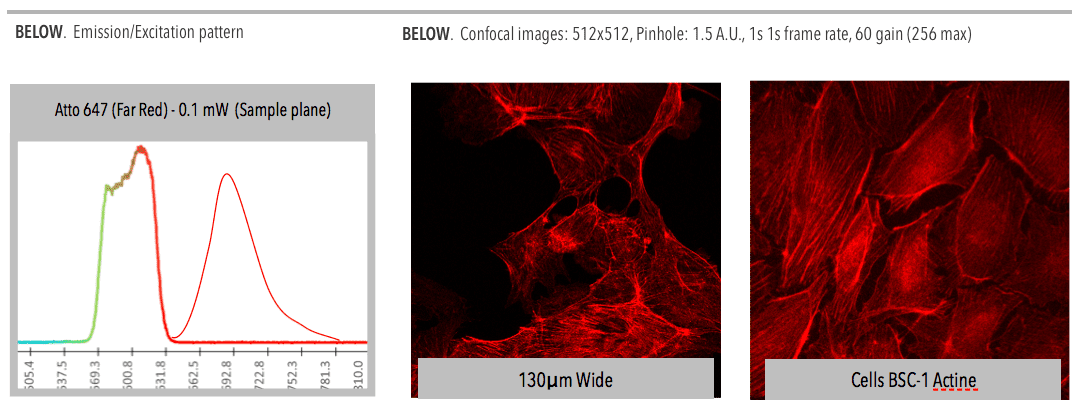
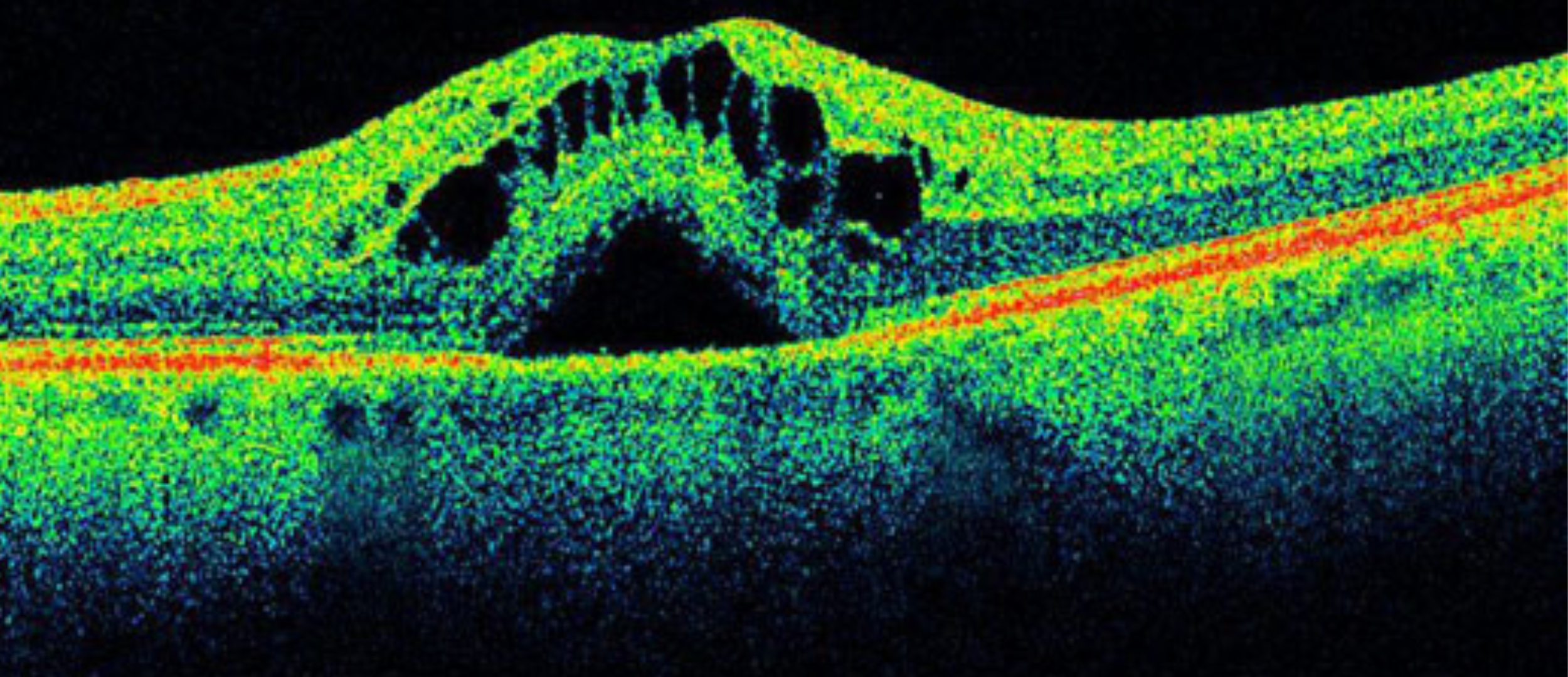
Confocal microscopy, most frequently confocal laser scanning microscopy (CLSM), is an optical imaging technique for increasing optical resolution and contrast of a micrograph by means of adding a spatial pinhole placed at the confocal plane of the lens to eliminate out-of-focus light.
Fine detail is often obscured by the haze and cannot be detected in a non-confocal, fluorescent microscope. The confocal microscope has a stepper motor attached to the fine focus, enabling the collection of a series of images through a three dimensional object.
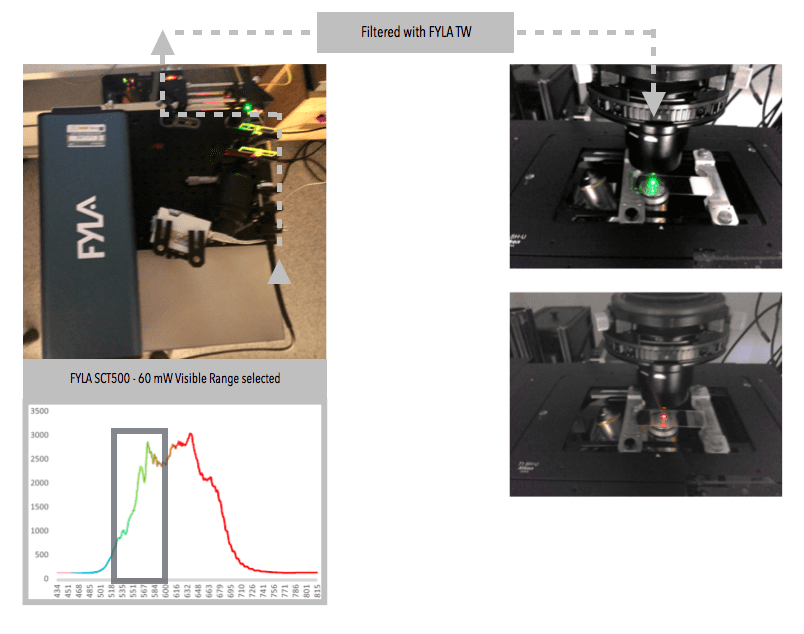
A real limitation of lasers is their monochromy, which requires a whole battery of lasers to be combined in order to perform multi-parameter fluorescence measurements in simultaneous mode, a method expected as a standard by biomedical researchers. These batteries will be replaced by an all finer single SCT500 FYLA laser.
Fine detail is often obscured by the haze and cannot be detected in a non-confocal, fluorescent microscope. The confocal microscope has a stepper motor attached to the fine focus, enabling the collection of a series of images through a three dimensional object.
A real limitation of lasers is their monochromy, which requires a whole battery of lasers to be combined in order to perform multi-parameter fluorescence measurements in simultaneous mode, a method expected as a standard by biomedical researchers. These batteries will be replaced by an all finer single SCT500 FYLA laser.